Non-invasive, revolutionary Technology for Tightening & Lifting Skin
The easier, more natural solution for facelifts & more
The non-invasive Ultherapy® procedure is U.S. FDA-cleared to lift skin on the neck, on the eyebrow and under the chin, as well as to improve lines and wrinkles on the décolletage. This nonsurgical alternative to a traditional facelift is proven to lifting sagging skin, with more natural-looking results and virtually no downtime. Using ultrasound and real-time visualization technology, this treatment allows our skin experts to precisely target and tighten skin on the face, neck and chest. Ultherapy® uses nonsurgical ultrasound energy that is able to bypass the skin and penetrate deep layers underneath, restimulating your skin’s natural collagen production. Ready to reverse the clock on your face, neck or décolletage with a noninvasive lift? Click here or give us a call to reserve your consultation today!

Slide title
Write your caption hereButton
Real Patients before & after treatment with Ultherapy®

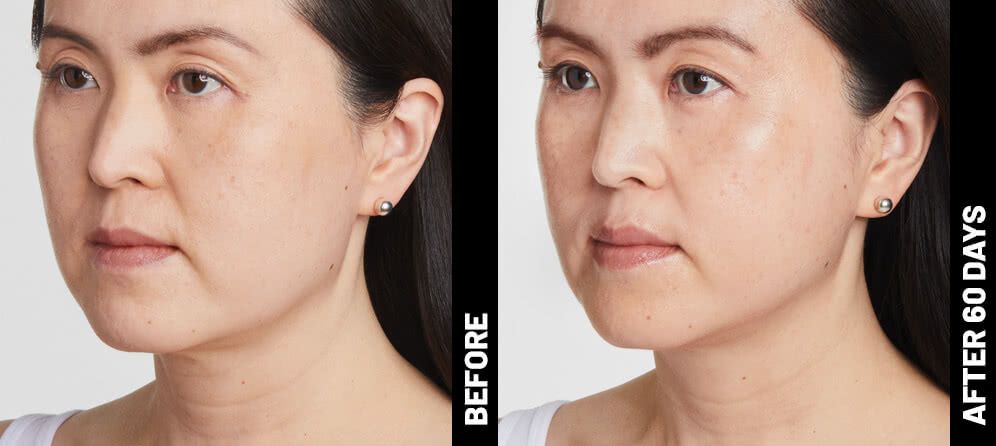
Slide title
Write your caption hereButton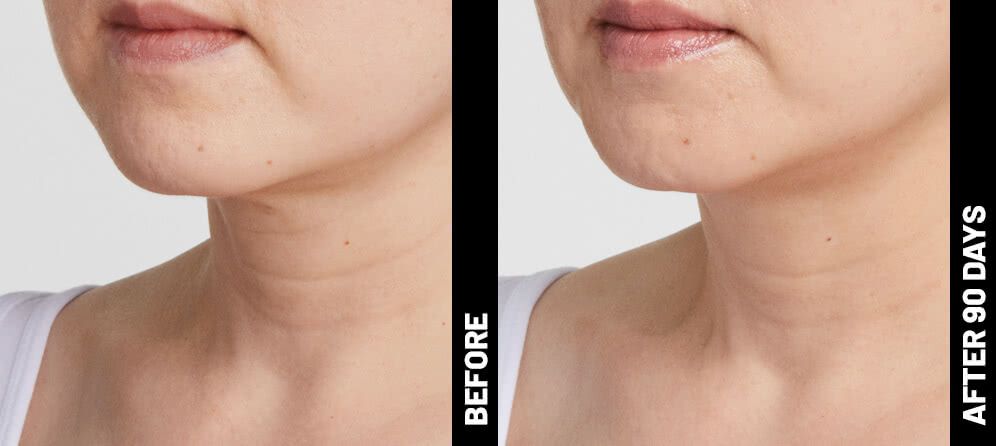
Slide title
Write your caption hereButton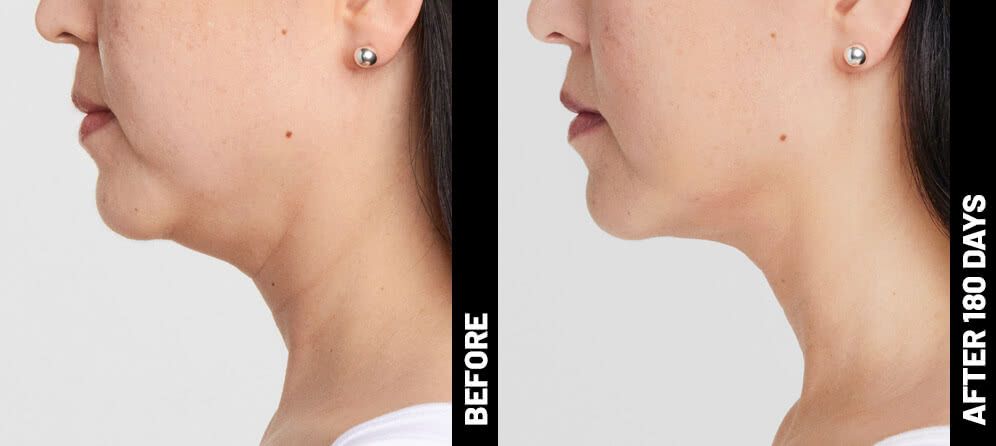
Slide title
Write your caption hereButton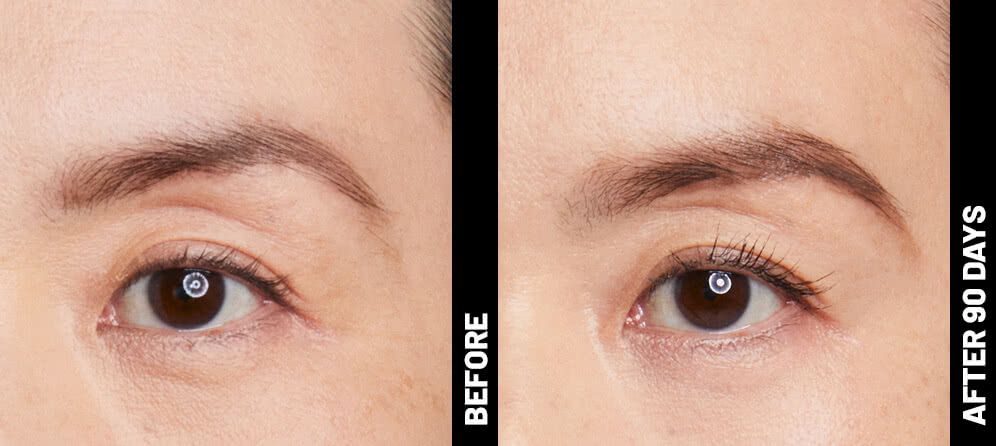
Slide title
Write your caption hereButton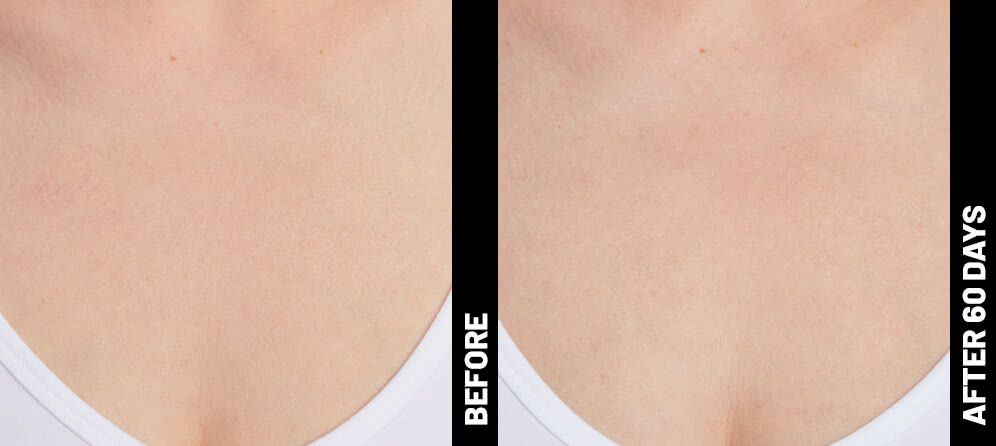
Slide title
Write your caption hereButton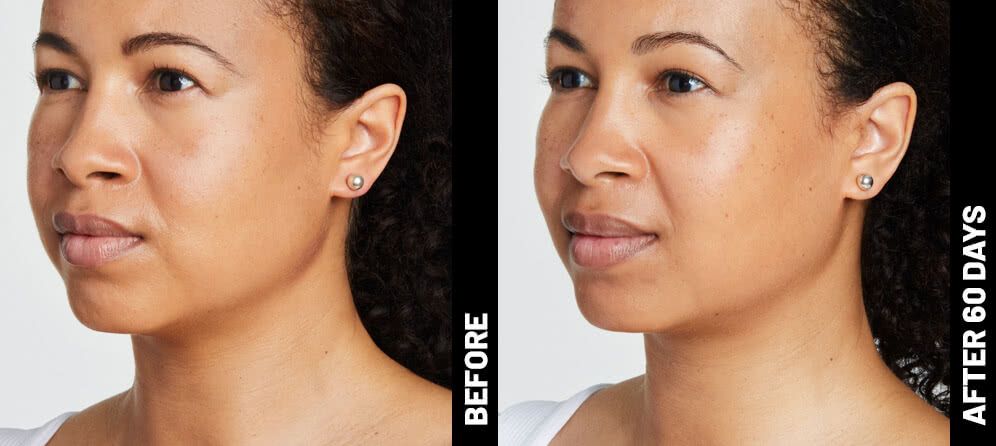
Slide title
Write your caption hereButton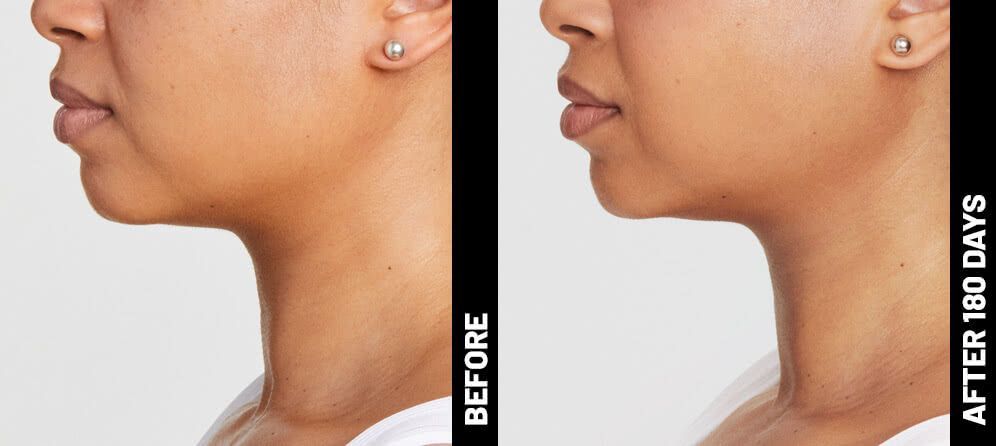
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton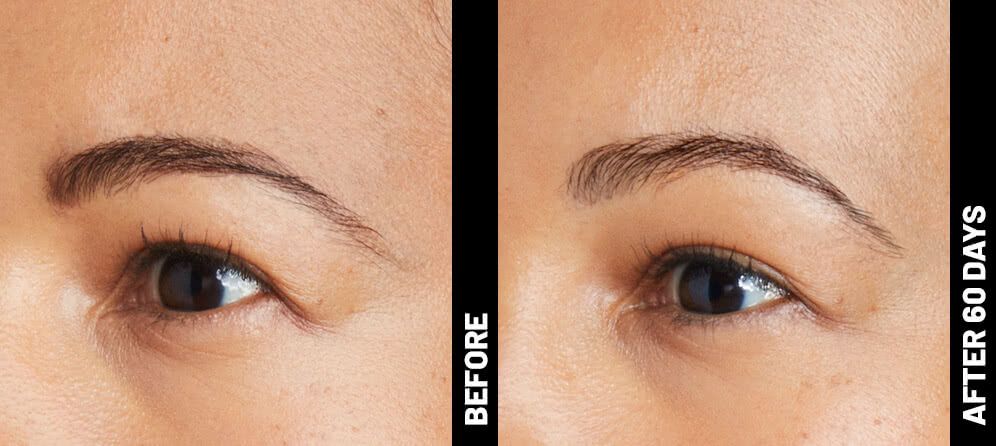
Slide title
Write your caption hereButton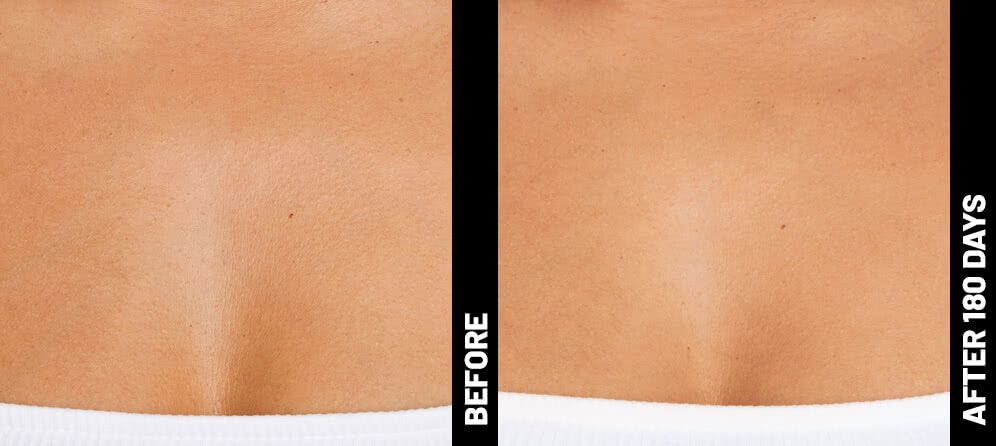
Slide title
Write your caption hereButton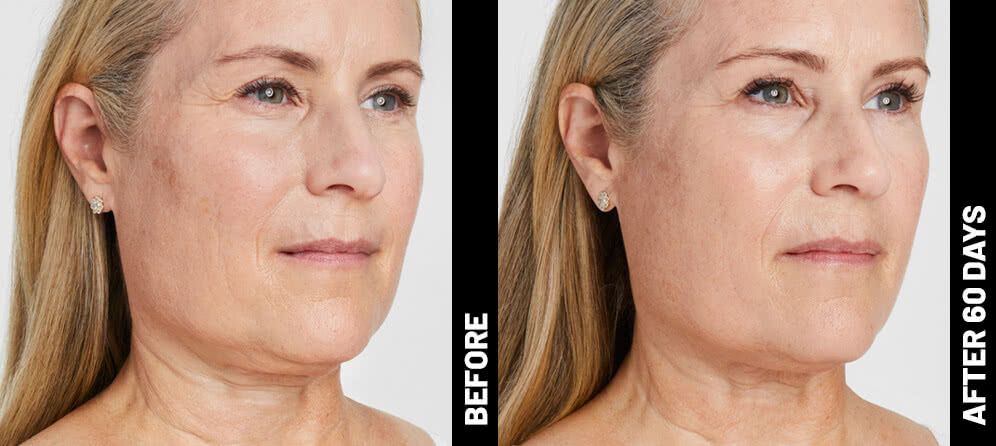
Slide title
Write your caption hereButton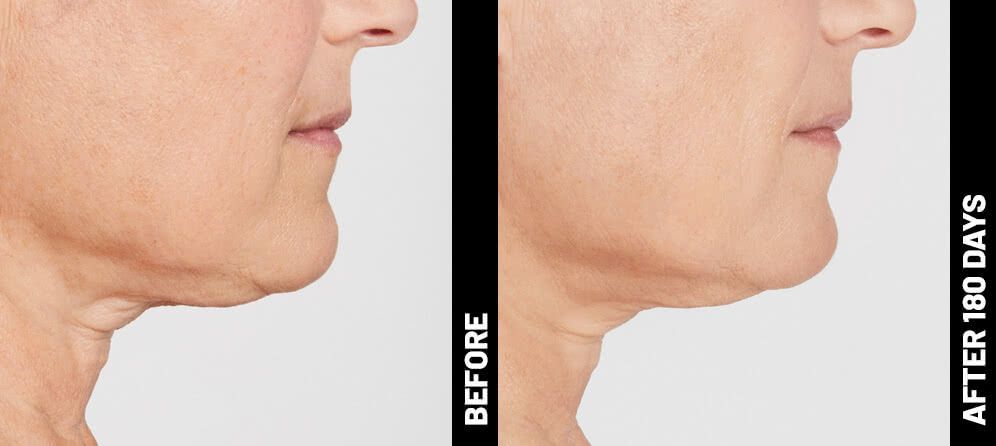
Slide title
Write your caption hereButton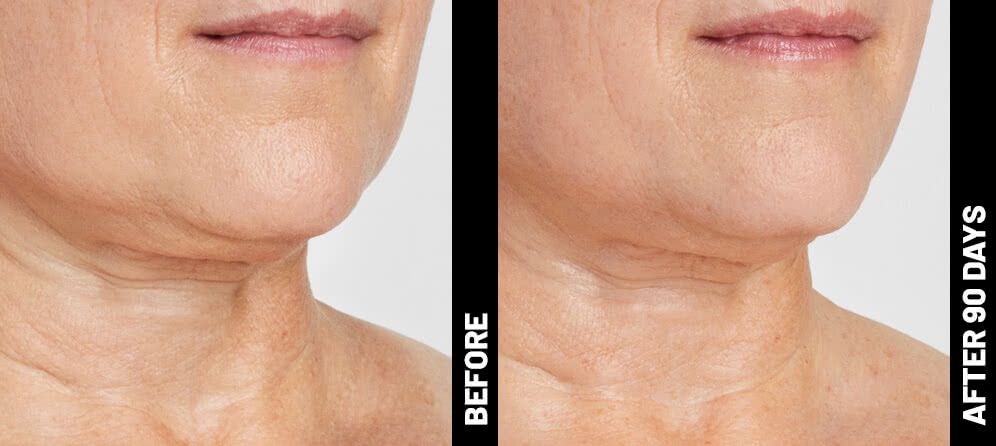
Slide title
Write your caption hereButton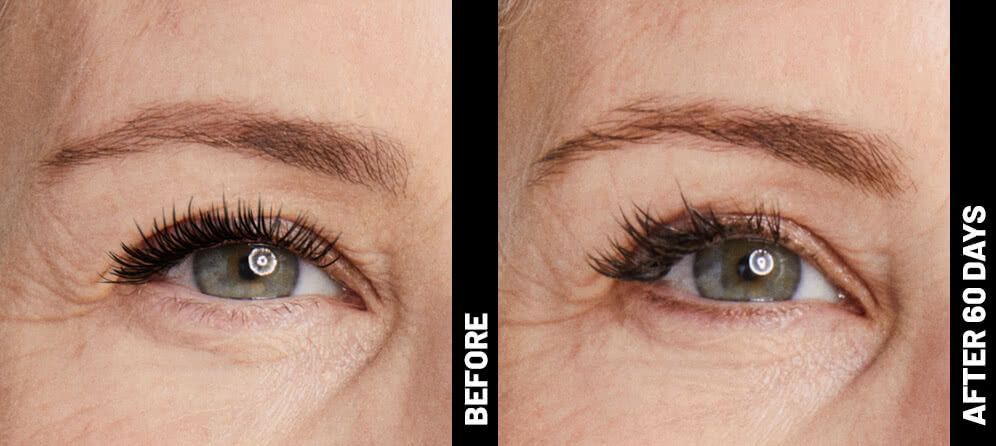
Slide title
Write your caption hereButton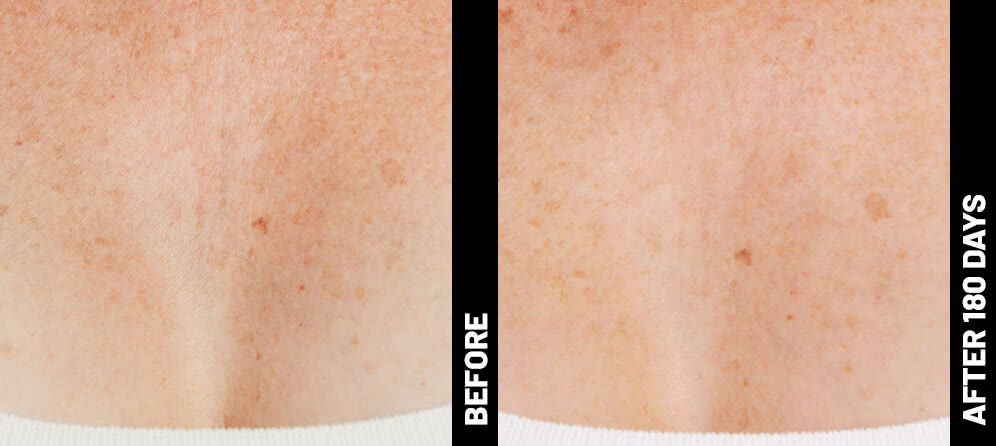
Slide title
Write your caption hereButton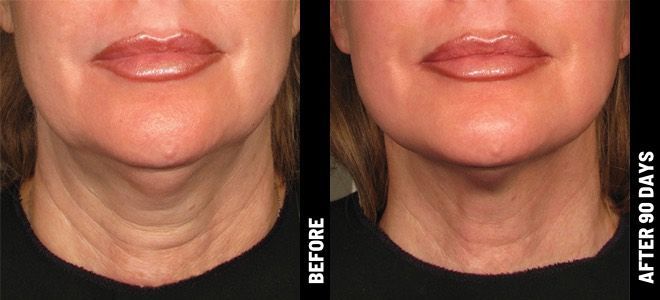
Slide title
Write your caption hereButton
-
IMPORTANT SAFETY INFORMATION - Ultherapy®
## **INDICATIONS FOR USE**
The Ulthera System is indicated for use as a non-invasive dermatological aesthetic treatment to:
- Lift the eyebrow
- Lift lax submental (beneath the chin) and neck tissue
- Improve lines and wrinkles of the décolletage
The Ulthera System, in conjunction with the Ulthera DeepSEE transducer, allows for ultrasonic visualization of depths up to 8 mm below the surface of the skin. The indicated use of the imaging is to visualize the dermal and subdermal layers of tissue to:
- Ensure proper coupling of the transducer to the skin
- Confirm appropriate depth of treatment such as to avoid bone
## **CONTRAINDICATIONS**
The Ulthera System is contraindicated for use in patients with:
- Open wounds or lesions in the treatment area
- Severe or cystic acne in the treatment area
- Active implants (e.g., pacemakers or defibrillators), or metallic implants in the treatment area
## **PRECAUTIONS**
When not in use by trained personnel, the Ulthera® System Access Key should be removed from the system to help prevent unauthorized use. Keep the Ulthera® System Access Key in a designated place accessible only to authorized and trained personnel.
The Ulthera® System has not been evaluated for use over various materials. Therefore, treatment is not recommended directly over those areas with any of the following:
- Mechanical implants
- Dermal fillers
- Breast implants
Treatment energy is not recommended for use directly on an existing keloid. The Ulthera System has not been evaluated for use in patients on an anticoagulant treatment plan. It is recommended that the following areas should be avoided during treatment:
- Thyroid gland, thyroid cartilage and trachea
- Major vessels
- Breast tissue or breast implants
The Ulthera System has not been evaluated for use in the following patient populations:
- Pregnant or breast feeding women
- Children
- Those with the following disease states
- A hemorrhagic disorder or hemostatic dysfunction
- An active systemic or local skin disease that may alter wound healing
- Herpes Simplex
- Autoimmune Disease
- Diabetes
- Epilepsy
- Bell’s Palsy
## **PATIENT SAFETY**
- Warning: Ulthera should not be used on a patient’s eyes or in a location or technique where ultrasound energy can reach the eye.
- Warning: Use this system only if you are trained and qualified to do so.
- Warning: If any problems occur during system operation, take immediate action(s): lift the transducer off the patient’s skin, press the See pushbutton on handle to discontinue treatment in progress, and/or press the red emergency Stop button to completely halt system operation.
## **POTENTIAL SIDE EFFECTS**
Side effects reported in the clinical evaluation of the Ulthera System for the brow, submental (under the chin) area and neck treatment(s) were mild and transient in nature. These were limited to:
- Erythema (redness): The treated area may exhibit erythema immediately following treatment. This typically resolves within a few hours of treatment.
- Edema (swelling): The treated area may exhibit mild edema following treatment. This typically resolves within 3 to 72 hours of treatment.
- Welting: The treated area may exhibit a localized area of linear visible edema following treatment. This typically resolves within a week.
- Pain: Momentary discomfort may be experienced during the procedure while energy is being deposited. Post procedure discomfort typically resolves within 2 hours and 2 days. Tenderness to the touch is also possible and typically resolves within 2 days to 2 weeks of treatment.
- Bruising: Mild bruising, which is caused by damage to soft tissue blood vessels, may occur occasionally and typically resolves within 2 days to 2 weeks of treatment.
- Nerve Effects:
- Transient local muscle weakness may result after treatment due to inflammation of a motor nerve. This typically resolves in 2 to 6 weeks of treatment.
- Transient numbness may result after treatment due to inflammation of a sensory nerve. This typically resolves in 2 to 6 weeks of treatment.
- Transient pain, paresthesia and/or tingling may be experienced. This typically resolves in 2 to 6 weeks of treatment.
No permanent injuries to facial nerves have been reported during clinical trials.
- Burns/Scarring: The possibility for burns, which may or may not result in permanent scar formation, may occur if incorrect treatment technique is used (e.g. tilting transducer, incorrect line spacing, gel pockets, etc. see section 7.3.4 & 7.3.5). Some scars may respond to medical treatment and resolve fully.
Side effects reported in the clinical evaluation of the Ulthera System for the décolleté treatment were mild and transient in nature. These were limited to:
- Erythema (redness): The treated area may exhibit erythema immediately following treatment. This typically resolves within a few hours of treatment.
- Edema (swelling): The treated area may exhibit mild edema following treatment. This typically resolves within 3 to 48 hours of treatment.
- Pain: Momentary discomfort may be experienced during the procedure while energy is being deposited. Post procedure discomfort typically resolves within 2 hours and 2 days. Tenderness to the touch is also possible and typically resolves within 2 days to 2 weeks of treatment.
- Welting: The treated area may exhibit a localized area of linear visible edema following treatment. This typically resolves within 1 day to 3 weeks of treatment.
- Raised area of edema: The treated area may exhibit a localized area of linear visible edema following treatment. This typically resolves within 1 day to 3 weeks of treatment.
- Bruising: Mild bruising, which is caused by damage to soft tissue blood vessels, may occur occasionally and typically resolves within 3 days and 3 weeks of treatment.
- Transient Sensory Nerve Effects (as a result of inflammation of the nerve):
- Paresthesia and/or numbness may be experienced and typically resolves within 4 days to 5 weeks of treatment.
- Tingling may result after treatment and typically resolves within 3 to 5 days of treatment
- Itching may result after treatment and typically resolves within 1 to 3 weeks of treatment.
No permanent injuries to facial nerves have been reported during clinical trials.
- Burns/Scarring: The possibility for burns, which may or may not result in permanent scar formation, may occur if incorrect treatment technique is used (e.g. tilting transducer, incorrect line spacing, gel pockets, etc. see section 7.3.4 & 7.3.5). Some scars may respond to medical treatment and resolve fully.
## **COMPLAINTS AND ADVERSE EVENTS**
No serious adverse events were observed during the clinical study evaluation of the Ulthera System. Ulthera follows MDR (Medical Device Reporting) rules for handling complaints and adverse events. Should an adverse event be suspected or reported, contact Ulthera, Inc. at 480-619-4069; for those outside the U.S., contact your local Ulthera representative.
## **POST MARKET SURVEILLANCE**
The following adverse events have been identified during routine clinical use following FDA clearance (post market) of the Ulthera system. Because they are reported voluntarily from a population of uncertain size it is not always possible to reliably estimate their frequency or establish a causal relationship to the Ulthera system. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to the Ulthera system: pain, burns, or burning sensation, edema/swelling, nodules, bruising, fat/volume loss, neuropathy, numbness, paresthesia, palsy, paresis, speech difficulty, muscle weakness, headache, migraine, visual change, skin sagging/drooping, asymmetry, erythema, welts, hives, rash, urticaria, pruritus, blistering, scarring, discoloration and hyperpigmentation.
NOTE: This document is for web purposes only. It has been condensed from its original version. For additional information, contact 1.877.ULTHERA.
DCN14786 IFU Rev. A
