Erase Those Fine Lines with Wrinkle-Relaxing Injectables
Are fine lines and wrinkles grabbing your attention in the mirror?
Erase those lines with Neuromodulator injectables, such as BOTOX® Cosmetic, Xeomin®, and Dysport®. These non-surgical cosmetic enhancements are used to correct signs of again by relaxing facial muscles that cause fine lines, wrinkles, crow’s feet, laugh and frown lines, gummy smiles, and dimpling chin. Create a polished youthful look today!
Reserve your appointment now.

Slide title
Write your caption hereButton

Slide title
Write your caption hereButton
Looking to prevent wrinkles and deeper lines from forming?
Ask us about Microbotox Treatment!
Also known as "Baby Botox®", the Microbotox treatment is suitable for women in their 20s to early 30s who do not yet have wrinkles or lines, and are looking to retain a youthful appearance. Targeted injections of Botox® or other neuromodulators at smaller doses helps to prevent deeper lines from forming. Prevention is the best medicine, and now you can combat the signs of aging that naturally occur as we age. Ask our aesthetic specialists about Microbotox today!
Real Patients before & after treatment with BOTOX® Cosmetic


Slide title
Write your caption hereButton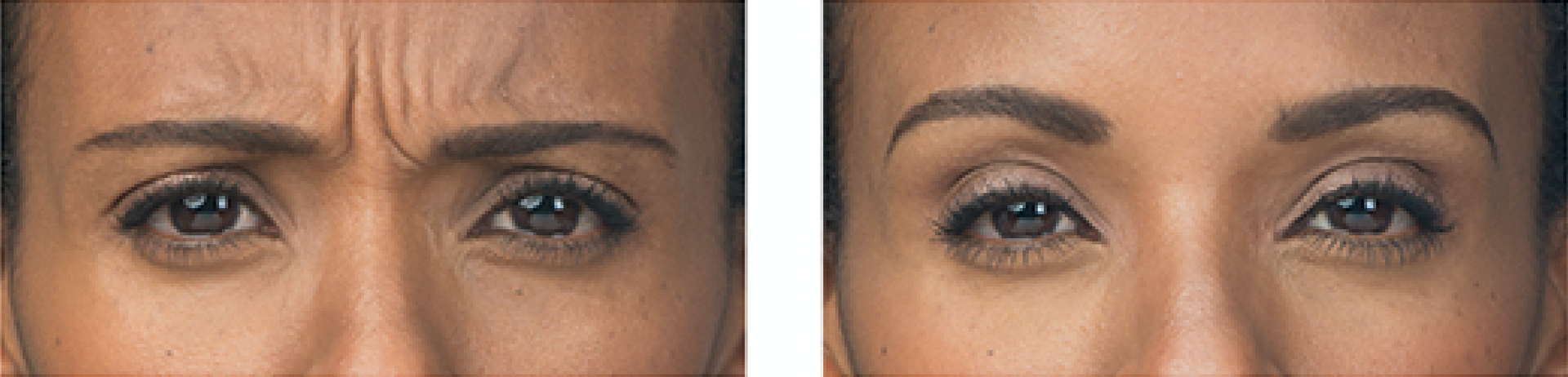
Slide title
Write your caption hereButton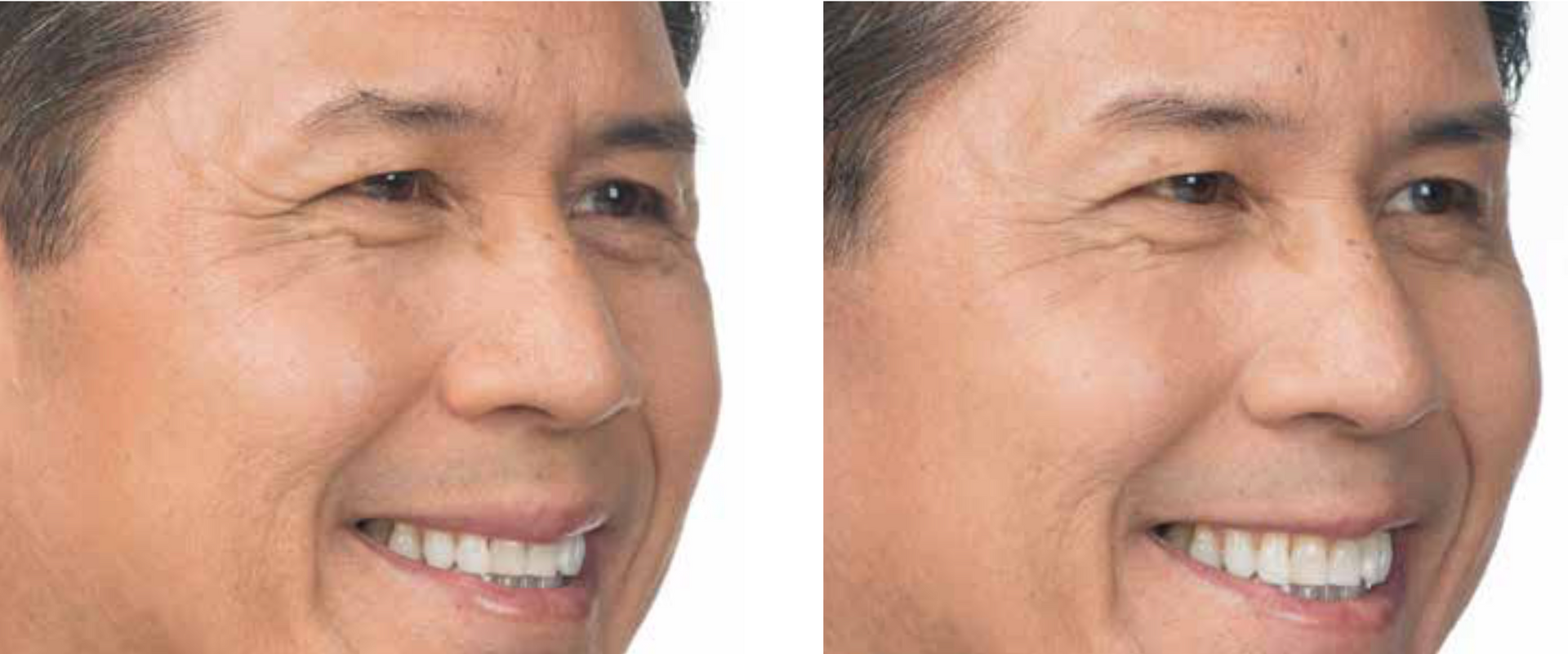
Slide title
Write your caption hereButton
Real Patients before & after treatment with XEOMIN®


Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
Real Patients before & after treatment with Dysport®

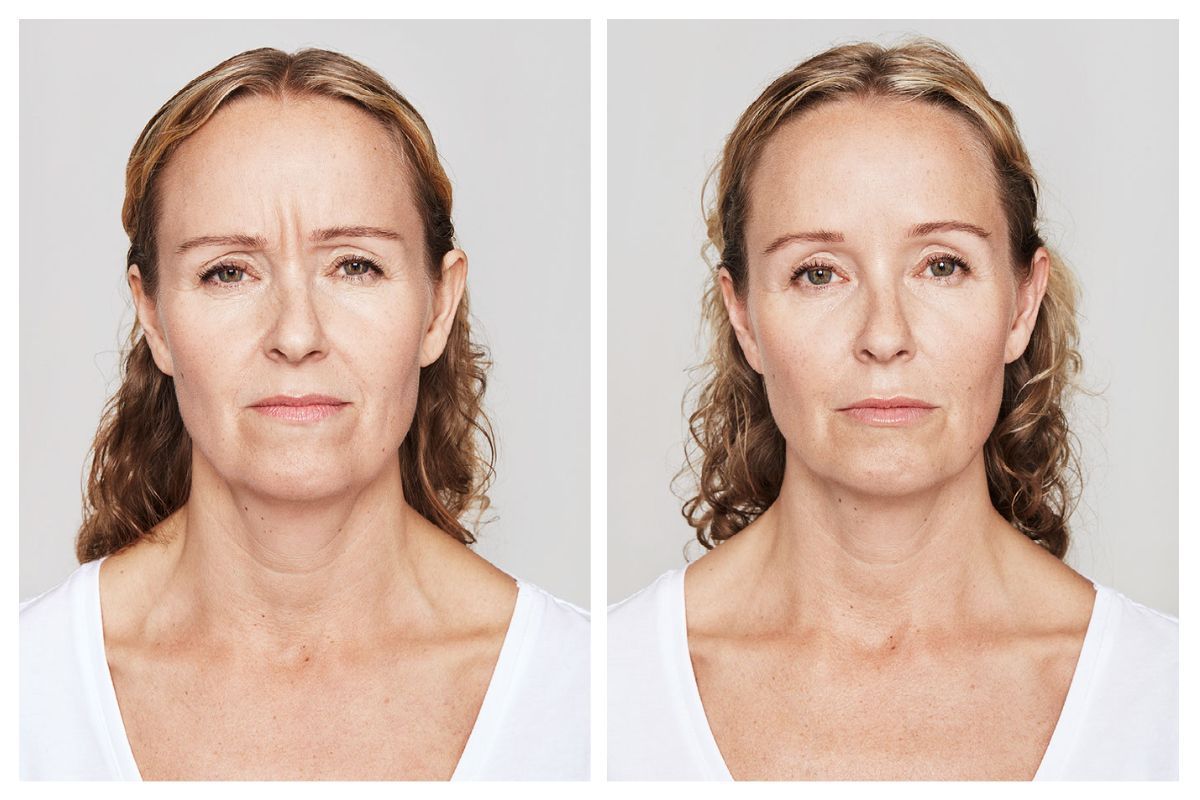
Slide title
Write your caption hereButton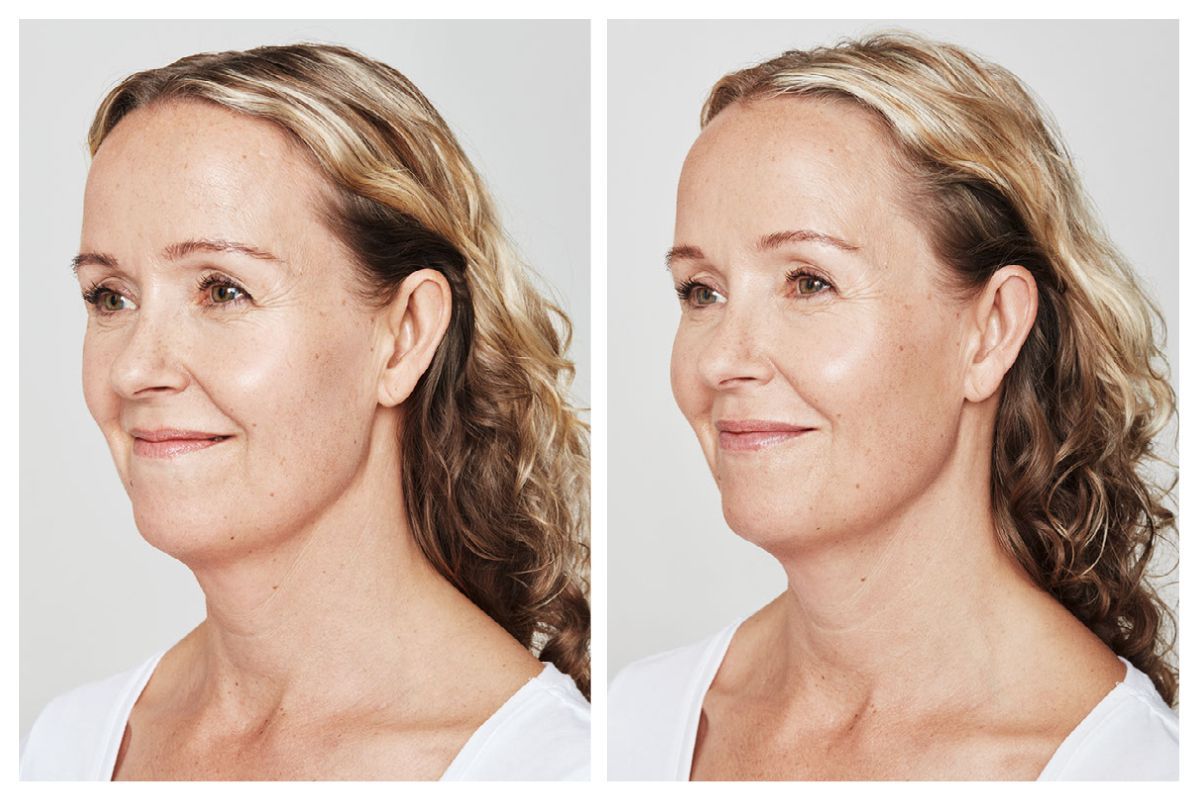
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
-
IMPORTANT SAFETY INFORMATION - BOTOX® Cosmetic
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
Indications
BOTOX® Cosmetic (onabotulinumtoxinA) is indicated in adult patients for the temporary improvement in the appearance of:
- moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
- moderate to severe lateral canthal lines associated with orbicularis oculi activity
- moderate to severe forehead lines associated with frontalis activity
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX® Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses, including spasticity in children, and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses.
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability between Botulinum Toxin Products
The potency Units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX® Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), and 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines) have been reported.
Serious Adverse Reactions With Unapproved Use
Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, with some adverse reactions associated with fatal outcomes, have been reported in patients who received BOTOX® injections for unapproved uses. In these cases, the adverse reactions were not necessarily related to distant spread of toxin, but may have resulted from the administration of BOTOX® to the site of injection and/or adjacent structures. In several of the cases, patients had pre-existing dysphagia or other significant disabilities. There is insufficient information to identify factors associated with an increased risk for adverse reactions associated with the unapproved uses of BOTOX®. The safety and effectiveness of BOTOX® for unapproved uses have not been established.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX® of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
Increased Risk of Clinically Significant Effects with Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including generalized muscle weakness, diplopia, ptosis, dysphonia, dysarthria, severe dysphagia, and respiratory compromise from onabotulinumtoxinA (see Warnings and Precautions).
Dysphagia and Breathing Difficulties
Treatment with BOTOX® and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or oropharyngeal muscles that control swallowing or breathing (see Boxed Warning).
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX® Cosmetic treatment is used in the presence of inflammation at the proposed injection site(s) or when excessive weakness or atrophy is present in the target muscle(s).
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
ADVERSE REACTIONS
The most frequently reported adverse reaction following injection of BOTOX® Cosmetic for glabellar lines was eyelid ptosis (3%).
The most frequently reported adverse reaction following injection of BOTOX® Cosmetic for lateral canthal lines was eyelid edema (1%).
The most frequently reported adverse reactions following injection of BOTOX® Cosmetic for forehead lines with glabellar lines were headache (9%), brow ptosis (2%) and eyelid ptosis (2%).
DRUG INTERACTIONS
Co-administration of BOTOX® Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like compounds) should only be performed with caution as the effect of the toxin may be potentiated. Use of anticholinergic drugs after administration of BOTOX® Cosmetic may potentiate systemic anticholinergic effects.
The effect of administering different botulinum neurotoxin products at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of BOTOX® Cosmetic.
USE IN SPECIFIC POPULATIONS
There are no studies or adequate data from postmarketing surveillance on the developmental risk associated with use of BOTOX® Cosmetic in pregnant women. There are no data on the presence of BOTOX® Cosmetic in human or animal milk, the effects on the breastfed child, or the effects on milk production.
-
IMPORTANT SAFETY INFORMATION - XEOMIN®
**XEOMIN® (incobotulinumtoxinA) IMPORTANT CONSUMER SAFETY INFORMATION**
Read the Medication Guide before you start receiving XEOMIN (Zeo-min) and each time XEOMIN is given to you as there may be new information. The risk information provided here is not comprehensive.
To learn more:
- Talk to your health care provider or pharmacist
- Visit www.xeominaesthetic.com to obtain the FDA-approved product labeling
- Call 1-866-862-1211
**Uses: XEOMIN is a prescription medicine that is injected into muscles and used to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults for a short period of time (temporary). It is not known if XEOMIN is safe and effective in children under 18 years of age. Please see additional Important Safety Information below and Full Prescribing Information and Medication Guide at XeominAesthetic.com.
**Warnings: XEOMIN may cause serious side effects that can be life threatening. Call your doctor or get medical help right away if you have any of these problems anytime (hours to weeks) after treatment with XEOMIN:**
- **Problems with swallowing, speaking, or breathing can happen within hours to weeks after an injection of XEOMIN** if the muscles that you use to breathe and swallow become weak. Death can happen as a complication if you have severe problems with swallowing or breathing after treatment with XEOMIN.
- People with certain breathing problems may need to use muscles in their neck to help them breathe and may be at greater risk for serious breathing problems with XEOMIN.
- Swallowing problems may last for several months, and during that time you may need a feeding tube to receive food and water. If swallowing problems are severe, food or liquids may go into your lungs. People who already have swallowing or breathing problems before receiving XEOMIN have the highest risk of getting these problems.
- Spread of toxin effects. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include: loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing.
These symptoms can happen hours to weeks after you receive an injection of XEOMIN. These problems could make it unsafe for you to drive a car or do other dangerous activities.
**Do not use XEOMIN** if you are allergic to XEOMIN or any of the ingredients in XEOMIN (see the end of this Guide for a list of ingredients in XEOMIN), had an allergic reaction to any other botulinum toxin products such as rimabotulinumtoxinB (MYOBLOC®), onabotulinumtoxinA (BOTOX®, BOTOX® COSMETIC), or abobotulinumtoxinA (DYSPORT®) or have a skin infection at the planned injection site.
**Before receiving XEOMIN, tell your doctor about all of your medical conditions, including if you:**
- have a disease that affects your muscles and nerves (such as amyotrophic lateral sclerosis [ALS or Lou Gehrig’s disease], myasthenia gravis or Lambert-Eaton syndrome)
- have had any side effect from any other botulinum toxin in the past
- have a breathing problem such as asthma or emphysema
- have a history of swallowing problems or inhaling food or fluid into your lungs (aspiration)
- have bleeding problems
- have drooping eyelids
- have plans to have surgery
- have had surgery on your face
- are pregnant or plan to become pregnant. It is not known if XEOMIN can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if XEOMIN passes into breast milk.
**Tell your doctor about all of the medicines you take**, including prescription and over-the-counter medicines, vitamins and herbal supplements. **Talk to your doctor before you take any new medicines after you receive XEOMIN**.
Using XEOMIN with certain other medicines may cause serious side effects. **Do not start any new medicines until you have told your doctor that you have received XEOMIN in the past. Especially tell your doctor if you**
- have received any other botulinum toxin product in the last four months
- have received injections of botulinum toxin such as rimabotulinumtoxinB (MYOBLOC), onabotulinumtoxinA (BOTOX, BOTOX COSMETIC) and abobotulinumtoxinA (DYSPORT) in the past. Be sure your doctor knows exactly which product you received. The dose of XEOMIN may be different from other botulinum toxin products that you have received.
- have recently received an antibiotic by injection
- take muscle relaxants
- take an allergy or cold medicine
- take a sleep medicine
**Ask your doctor if you are not sure if your medicine is one that is listed above.** Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
**Possible Side Effects**
**XEOMIN can cause serious side effects that can be life threatening including** allergic reactions. Symptoms of an allergic reaction to XEOMIN may include: itching, rash, redness, swelling, wheezing, asthma symptoms, or dizziness or feeling faint. Tell your doctor or get medical help right away if you get wheezing or asthma symptoms, or if you get dizzy or faint. **See “Warnings.”**
**The most common side effect of XEOMIN in people with frown lines** include:
- headache
**These are not all the possible side effects of XEOMIN.** Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
**General information about the safe and effective use of XEOMIN**
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or doctor for information about XEOMIN that is written for health professionals.
**Active Ingredient**: botulinum toxin type A
**Inactive Ingredients**: human albumin and sucrose
Copyright © 2018 Merz North America, Inc. All rights reserved.
-
IMPORTANT SAFETY INFORMATION - Dysport®
Dysport® (abobotulinumtoxinA) is a prescription injection for temporary improvement in the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults less than 65 years of age.
Important Safety Information
What is the most important information you should know about Dysport? Spread of Toxin Effects: In some cases, the effects of Dysport and all botulinum toxin products may affect areas of the body away from the injection site. Symptoms can happen hours to weeks after injection and may include swallowing and breathing problems, loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, or loss of bladder control. Swallowing and breathing problems can be life threatening and there have been reports of death. You are at the highest risk if these problems are pre‐existing before injection.
These effects could make it unsafe for you to drive a car, operate machinery, or do other dangerous activities.
Do not have Dysport treatment if you: are allergic to Dysport or any of its ingredients (see the end of the Medication Guide for a list of ingredients), are allergic to cow’s milk protein, had an allergic reaction to any other botulinum toxin product, such as Myobloc®, Botox®, or Xeomin®, have a skin infection at the planned injection site, under 18 years of age, or are pregnant or breastfeeding.
The dose of Dysport is not the same as the dose of any other botulinum toxin product and cannot be compared to the dose of any other product you may have used.
Tell your doctor about any swallowing or breathing difficulties and all your muscle or nerve conditions such as amyotrophic lateral sclerosis [ALS or Lou Gehrig’s disease], myasthenia gravis, or Lambert‐Eaton syndrome, which may increase the risk of serious side effects including difficulty swallowing and difficulty breathing. Serious allergic reactions have occurred with the use of Dysport. Dry eye has also been reported.
Tell your doctor about all of your medical conditions, including if you have surgical changes to your face, very weak muscles in the treatment area, any abnormal facial change, injection site inflammation, droopy eyelids or sagging eyelid folds, deep facial scars, thick oily skin, wrinkles that can’t be smoothed by spreading them apart, or if you are pregnant or breastfeeding or planning to become pregnant or breastfeed.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal and other natural products. Using Dysport with certain other medicines may cause serious side effects. Do not start any new medicines while taking Dysport without talking to your doctor first.
Especially tell your doctor if you: have received any other botulinum toxin product, such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA), or Xeomin® (incobotulinumtoxinA), in the last four months or any in the past (be sure your doctor knows exactly which product you received), have recently received an antibiotic by injection, take muscle relaxants, take an allergy or cold medicine, or take a sleep medicine.
Common Side Effects
The most common side effects are nose and throat irritation, headache, injection site pain, injection site skin reaction, upper respiratory tract infection, eyelid swelling, eyelid drooping, sinus inflammation, and nausea.
Ask your doctor if Dysport is right for you.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Dysport Full Prescribing Information including Medication Guide at DysportUSA.com.
